
-
Phone+81-3-6712-5985
- Contact Us
Phone+81-3-6712-5985

Welcome to S-Cube’s Pharma Patent Strategy Blog. We hope this space will serve as a vehicle to provide essential knowledge on patent strategy and highlight the most recent and impactful topics from both the brand and generic perspectives. We aim to deliver insights that elevate patent strategy understanding and provide practical points of reference not just for those working in the pharmaceutical industry, but also those in academia and the investment community.
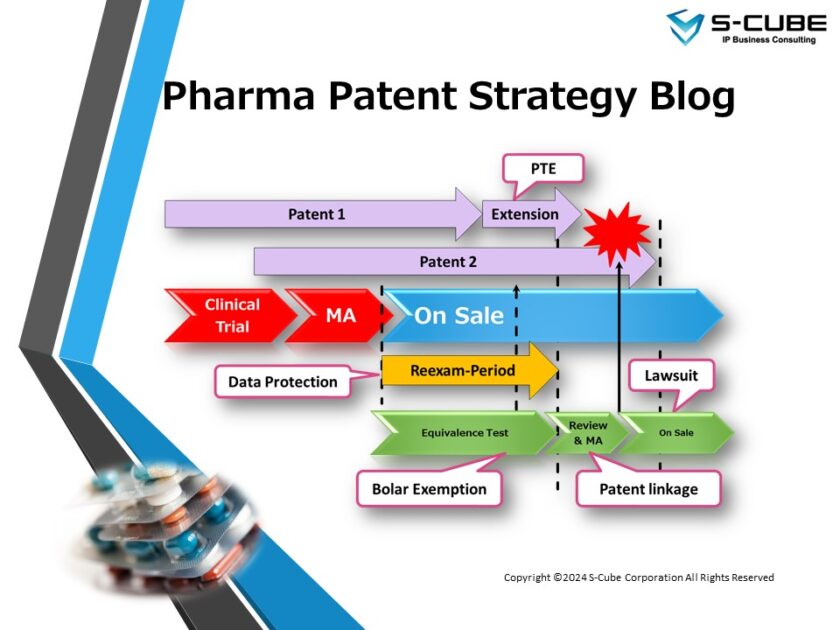
Today’s topic is “Operation of Patent Linkage in Japan.”
Japan has no laws or regulations governing patent linkage. However, based on two notifications from the Ministry of Health, Labor and Welfare (MHLW), patent linkage is implemented in two stages: First Stage is held in the review of generic drugs, and Second Stage is held after marketing approval (MA) but before listing on the NHI Drug Price Standard between the brand and generic makers.
The first Notice is Medical Affairs and Regulation No. 0605001 and NHI Drug Evaluation No. 0605014, both dated June 5, 2009, and jointly notified from two division directors of the MHLW (commonly known as “Joint Notice from Two Division Directors”, in Japanese known as “Nikacho-Tsuuchi (二課長通知)”). The Notice indicates that, from the perspective of ensuring a stable supply of pharmaceuticals, the Pharmaceuticals and Medical Devices Agency(PMDA) must check if there is an infringement of brand patents (substance and use patents) during the approval review of generic drugs. In other words, this Notice is the basis for patent linkage (First Stage) in the review of generic drugs.
The Joint Notice from Two Division Directors partially revises the one dated October 4, 1994 (“Handling of Pharmaceutical Patent Information in Relation to Applications for Approval”, YAKU-SHIN No. 762, dated October 4, 1994) and it seems that patent linkage had been in effect since this 1994 notice. The Joint Notice from Two Division Directors clarified that use patents are to be considered in addition to substance patents of brand drugs.
Furthermore, the Joint Notice from Two Division Directors also includes a provision that a generic drug can be approved with partial patent-free indication (curving-out, in Japan it is so-called “insect-eaten application”) if patents exist only for some indications of the brand drug.
The second Notice is Medical Affairs and Economics Bulletin No. 0115001, dated January 15, 2009.
This Notice suggests that, in order to ensure a stable supply of pharmaceuticals, if a party wishes to list an approved generic drug for which there is a possibility of patent disputes before the generic drug is listed in the NHI Drug Price Listing, the parties concerned should negotiate, and only the one for which a stable supply is expected to be available should be listed. In other words, the Notice is the basis for patent linkage after the MA and before listing on the NHI Drug Price Standard (Second Stage, negotiation before Drug Price listing).
The First and Second Stages of patent linkage are as follows.
First stage (Generic Drug Review : Application — Review — MA)
Once a generic drug application is submitted to the MHLW, it is reviewed by the PMDA and approved in about one year. The “Joint Notice from Two Division Directors,” which is the basis for the First Stage of patent linkage, states that during the review process, it is confirmed that “the submitted generic drug does not conflict with the brand patents. The brand patents refer to substance/use patents described in “Pharma Patent Information Sheet.”
The Pharma Patent Information Sheet is one of the submission documents when a brand company files an application for a new drug and includes brand patents. In the US, “Orange Book” includes brand patents. While the Orange Book is publicly available, the Pharma Patent Information Sheet is not. The patent information in the Pharma Patent Information Sheet can be revised or added up to the end of the reexamination period. (“Reexamination period” is, similar to or Japanese version of the data-protection period, a period of eight years for new drugs (Article 14-4 of the Pharmaceutical and Medical Device Act)) The submission of a Pharma Patent Information Sheet and the inclusion of any patent information is highly recommended to submit it but not mandatory.
According to the Joint Notice from Two Division Directors, the First Stage of patent linkage is to check the relationship between the substance/use patents listed in the Pharma Patent Information Sheet and the generic drug, and other patents (e.g., formulation patents, manufacturing process patents) are not referenced at this stage. And as mentioned above, there are no regulations regarding the submission of the Pharma Patent Information Sheet and its contents, so there is no penalty even if there is an omission in the substance/use patents or if patents other than substance/use patents are listed.
In addition, although not specified in the Joint Notice from Two Division Directors, the patent invalidation trial decision and the court decision are listed as “evidence showing that the generic drug can be manufactured/imported and sold promptly after MA” that should be attached to the generic drug application, so it is assumed that validity of the brand patent is also reviewed as well as infringement in the First Stage (Katsumi Shinohara, “Various Issues of the Japanese Patent Linkage System,” L&T 80, 29-35 (2018)).
The Joint Notice from Two Division Directors says that the relation of the brand patent and generic drug is determined based on the expected MA date of the generic drug. Namely, if a brand patent is pending in a patent invalidation trial, the generic drug is not supposed to be approved unless the case is finalized on the scheduled MA date.
Here, in general, a decision of the patent invalidation trial in the JPO can be appeal to the Intellectual Property High Court of Japan and then to the Supreme Court. Considering the cases in which a remand to a lower court is ordered, it could take a while from the first decision in the JPO until the case is finalized due to the repetition of the appeals and returns. Therefore, even if a generic drug is applied, it could not be approved for a considerable period due to the invalidation trial not being finalized.
Second Stage (Negotiation before Drug Price Listing: MA — NHI Price Listing)
After a generic MA has been granted , it is necessary to be listed in the NHI Drug Price Standard (registration of drug prices) to marketize an ethical drug. Those who have received MA must apply for listing on the NHI Drug Price Standard within a certain period.
Generic drugs are approved twice a year (February and August), and the NHI drug price standard listing also occurs twice a year (June and December), so that a generic drug approved in February can be listed on the NHI drug price standard in June of the same year at the earliest, or in December if approved in August. When a generic drug is approved, the MA information is posted on the NHI website, and this is how the brand maker becomes aware of the generic drug’s existence.
When an expected MA date of generic drugs is approaching, the PMDA notifies member companies of the NHI through the Federation of Pharmaceutical Manufacturers’ Associations of JAPAN (FPMAJ: http://www.fpmaj.gr.jp/ ) that if they wish to list the generic drug at the NHI drug price that is likely to be the subject of a patent dispute, they must negotiate before Drug Price listing and submit the results to the MHLW by a specified date (Second Stage: Negotiation before Drug Price Listing).
The substance and use patents are supposed to be reviewed during the First Stage of patent linkage, so the Second Stage is to check for infringement of other patents such as formulation and manufacturing process patents. Normally, when a brand maker finds a generic drug approved that possibly infringes their patents, they will contact the generic maker directly to initiate negotiations before the Drug Price listing. Then both parties must report to the MHLW at the beginning and end of the negotiation before the Drug Price listing period. Although reporting to the MHLW is mandatory, it is not necessary for both parties to have reached an agreement, as it is only necessary to make a report. Therefore, even if the NHI drug price is listed after negotiation before the Drug Price listing, it does not mean that there is no patent infringement or lawsuit. A patent lawsuit may occur after the NHI drug price is listed, or it may also occur before the NHI drug price is listed to prevent drug price listing.
In the case of additional MA, such as new indication or dose and dosage, for a generic drug that has already been initially approved and the NHI price has already been listed, the drug can be sold right after the MA (because the generic drug has been priced). In such cases, there is no chance of Second Stage of patent linkage.