
-
Phone+81-3-6712-5985
- Contact Us
Phone+81-3-6712-5985

Welcome to S-Cube’s Pharma Patent Strategy Blog. We hope this space will serve as a vehicle to provide essential knowledge on patent strategy and highlight the most recent and impactful topics from both the brand and generic perspectives. We aim to deliver insights that elevate patent strategy understanding and provide practical points of reference not just for those working in the pharmaceutical industry, but also those in academia and the investment community.
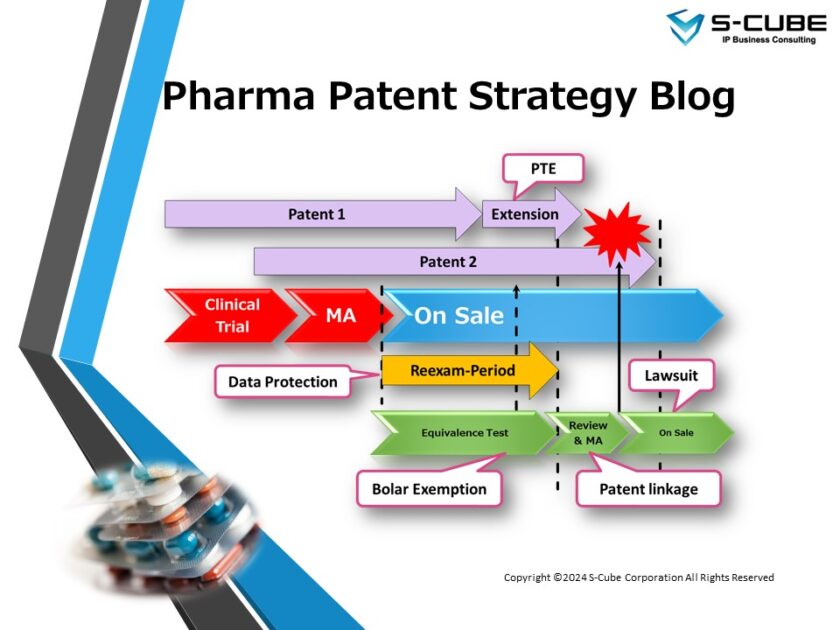
This time, we will be looking at “Patent Linkage in the United States”
Overview of Patent Linkage in the United States
Patent linkage in the United States is stipulated in the so-called Hatch-Waxman Act (21 U.S.C. § 505(j): Drug Price Competition and Patent Term Restoration Act). This law was drafted by Republican Senator Orrin Hatch and Democratic Representative Henry Waxman in 1984 with the aim of promoting the development of the US pharmaceutical industry as a whole by striking a balance between the interests of the original and generic drug manufacturers [1].
Against this background, the Hatch-Waxman Act not only covers patent linkage, but also sets out provisions on the Abbreviated New Drug Application (ANDA) system for generic drugs, the patent term extension system (35 U.S.C. §156), and the exception for experimental and research purposes (35 U.S.C. §271(e)(1): Bolar exemption).
Please note that the patent linkage stipulated in the Hatch-Waxman Act applies to small-molecular-weight pharmaceuticals. For biopharmaceuticals, the patent linkage stipulated in the BPCIA (Biologics Price Competition and Innovation Act) “patent dance” applies.
ANDA
ANDA is an abbreviation for Abbreviated New Drug Application, which translates into Japanese as “後発医薬品の簡易申請”. ANDA is stipulated in the aforementioned Hatch-Waxman Act. Prior to the Act, even generic drugs were required to undergo the same clinical trials as new drugs. For this reason, the cost benefits of generic drugs were far smaller than they are today.
When applying for an ANDA, the applicant must submit a certificate stating the relationship with the original patent listed in the Orange Book in addition to proving bioequivalence with the original drug. The certificate should be one of four types (Paragraphs I to IV, see below) depending on the relationship with the original patent.
• Paragraph I: No brand patent is listed in the Orange Book
• Paragraph II: The patent has expired or has been revoked
• Paragraph III: The applied generic drug is scheduled to be released after the patent expires
• Paragraph IV: The listed patent is invalid, unenforceable, or non-infringing
Applications with either a Paragraph I or II certificate will be approved if other requirements are met. In the case of Paragraph III, approval will be granted after the patent expires.
In the case of an application with a Paragraph IV certificate, the applicant must notify the holder of the NDA (New Drug Approval) and the patent holder within 20 days of submission (Notice Letter). This leads to so-called ANDA litigation.
However, if the indication for which the ANDA applicant is seeking approval differs from the use (purpose) of the patent listed in the Orange Book, a “Section VIII Statement” can be submitted (carve-out), and notice is not required (21 U.S.C. §355(j)(2)(A)(viii)). It is probably safe to assume that “carve-out” is equivalent to the Japanese term “虫食い申請 (bug-bite)”.
ANDA litigation (Hatch-Waxman litigation)
The Notice Letter must include a detailed statement of the facts and legal basis supporting the applicant’s position on the validity and non-infringement of the patent.
If the Paragraph IV Certification asserts that the patent is invalid or unenforceable, a full and detailed explanation of the evidence supporting the assertion is required for each claim asserted as invalid or unenforceable. On the other hand, if the certificate asserts non-infringement, the reasons for no infringement for each claim need to be explained.
In order for the Paragraph IV Certificate to claim non-infringement of the patent and for the ANDA applicant to later file a declaratory judgment action, a request for disclosure of the application documents must be attached to the Notice Letter.
The patent holder has 45 days from receipt of the Notice Letter to file a patent infringement lawsuit against the ANDA applicant. This is known as an ANDA lawsuit or a Hatch-Waxman lawsuit.
Once a lawsuit is filed, the ANDA approval process is suspended for 30 months (30-month stay).
Therefore, the NDA holder and the patent holder (the innovator) can secure the benefit of not having the generic drug approved for 30 months.
In addition, if a lawsuit is filed, the ANDA applicant must notify the FDA in writing within 14 days of the lawsuit date.
The lawsuit, including the invalidation of the patent, will be heard by a judge in court.
If a decision is made to invalidate the patent or to find non-infringement within 30 months of the start of the litigation, or if the case is not concluded within 30 months, the ANDA will be approved after 30 months. If the patent holder does not file a lawsuit, the FDA will review and approve the ANDA. The first ANDA approval holder will be granted a 180-day period of exclusivity.
Declaratory Judgment Action by ANDA Applicant
After the Notice Letter to the ANDA applicant, if the patent holder does not file a lawsuit within 30 months, the ANDA applicant may file a declaratory judgment action against the innovator to establish that the patent listed in the Orange Book is invalid or that the drug for which the ANDA was filed does not infringe the patent listed in the Orange Book (21 U.S.C. §355(j)(5)(C)(i)).
If the patent holder does not file an ANDA lawsuit (i.e. independently of patent linkage), they can file an infringement lawsuit based on the patent listed in the Orange Book. It is also possible to file a lawsuit based on a patent not listed in the Orange Book, independently of patent linkage.
Therefore, a declaratory judgment action is effective for ANDA applicants who are concerned that a separate lawsuit may be filed if an ANDA lawsuit is not filed. However, even if a declaratory judgment action is filed, the ANDA approval process will not be suspended.
As a requirement for filing a declaratory judgment action, the ANDA applicant must submit a written authorization to access confidential information contained in the application (21 U.S.C. §355(j)(5)(C)(i)(I)(cc)).
Footnote: [1] Toshihiko Asano, “Trends in the US Pro-Patent Policy in the Pharmaceutical and Biotechnology Fields – Focusing on the Hatch-Waxman Act-,” Chizai-ken Kiyo, 121-122 (2006)